| |
 |
CATALYSIS AND SUPRAMOLECULAR CHEMISTRY
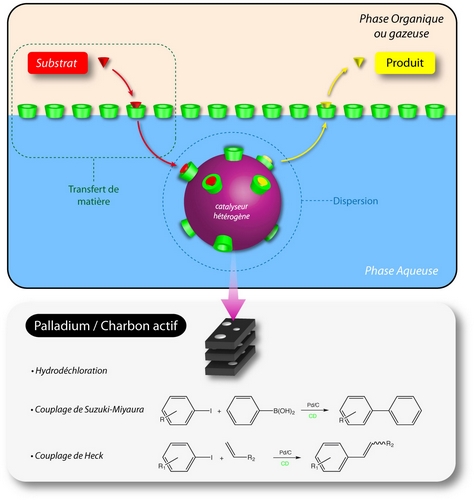
Pd/charcoal catalysts
We have clearly established the efficiency of chemically modified CDs (especially methylated) in three different reactions catalyzed by palladium on charcoal in water: i) the hydrodechlorination (model reaction chosen for the development of remediation processes of chlorinated volatile organic compounds (VOCs) by adsorption-catalysis [P3]. i) the Suzuki-Miyaura reaction [P1] and iii) the Heck reaction [P5]. Beyond the gains in activity, we also demonstrated that methylated CDs generate interesting effects on the selectivity in hydrodechlorination of CCl4 and in C-C coupling Heck reaction. For the synthesis of ethyl cinnamate in the presence of methylated CDs, the amounts of by-products (homo-coupling and dehalogenation) significantly decreased when the E/Z ratio increased. These variations have been attributed to the change in the local environment of the palladium sites on the charcoal surface.
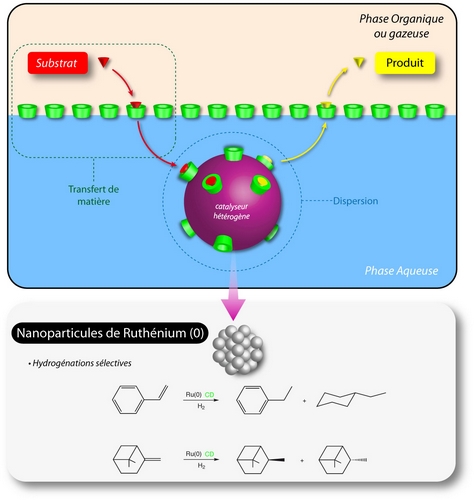
Stabilized ruthenium-nanoparticles
We discovered that methylated CDs are able to stabilize ruthenium colloidal suspensions in water, the obtained nanoparticles having an average diameter of 1 to 2,5 nm [P2]. This property has been turned to account to develop catalytic colloidal species active in hydrogenation of aromatic compounds and pinene in water. In these systems, the chemically modified CDs appeared to be multi-functional entities during the course of the reaction [P2, P4]. Actually, they stabilize the catalytic species, they improve the transfer of the substrate to the catalytic site and finally they orient the regio- or stereo-selectivity according to the methylation degree or the size of the CD cavity. These properties ensue from the recognition process between the substrate and the CD hydrophobic cavity.
Publications :
- P 1. Unexpected multi-functional effects of methylated cyclodextrins in palladium charcoal-catalyzed Suzuki-Miyaura reaction.
A. Cassez, A. Ponchel, F. Hapiot, E. Monflier, Org. Lett. 8 (2006) 4823-4826.
- P 2. Supramolecular shuttle and protective agent: a multiple role of methylated cyclodextrins in the chemoselective hydrogenation of benzene derivatives with ruthenium particles.
A. Nowicki, Y. Zhang, B. Léger, J.P. Rolland, H. Bricout, E. Monflier, A. Roucoux, Chem. Commun. (2006) 296-298.
- P 3. Eco-efficient catalytic hydrodechloration of carbon tetrachloride in aqueous cyclodextrin solutions.
A. Cassez, A. Ponchel, H. Bricout, S. Fourmentin, D. Landy, E. Monflier,
Catal. Lett. 108 (2006) 209-214.
- P 4. Methylated Cyclodextrins: an efficient protective agent in water for zerovalent ruthenium nanoparticles and a supramolecular shuttle in alkene and arene hydrogenation reactions.
A. Nowicki-Denicourt, A. Ponchel, E. Monflier, A. Roucoux,
Dalton Trans. (2007) 5714-5719.
- P 5. Chemically modified cyclodextrins adsorbed on Pd/C particles : New opportunities to generate highly chemo- and stereoselective catalysts for Heck reaction.
A. Cassez, N. Kania, F. Hapiot, S. Fourmentin, E. Monflier, A. Ponchel,
Catal. Commun. 9 (2008) 1346-1351.
|
 |