



 |
 |
|||||||||||
 |
||||||||||||
| Home > Artois Teams > Catalysis and Supramolecular Chemistry > Aqueous organometallic catalysis > Elaboration of catalytic systems without cyclodextrin / phosphine association | ||||||||||||
CATALYSIS AND SUPRAMOLECULAR CHEMISTRYElaboration of catalytic systems avoiding
|
||||||||||||
 |
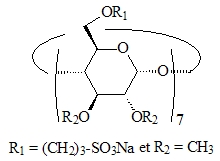 |
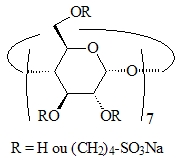
Figure 3 : anionic β-CD | Figure 4 : anionic methylated β-CD |
We then synthesized the heptakis(2,3-di-O-methyl-6-O-sulfopropyl)β-CD (Figure 4), an anionic CD having surface active properties. Interestingly, the absence of interaction with TPPTS is a consequence of the permethylation on the CD secondary face and does not result from electrostatic repulsions. In terms of activity, this CD is much more efficient than Rame-β-CD. From a selectivity point of view, the intrinsic properties of the catalytic system are fully preserved as the chemo- and regio-selectivities are identical to those observed without mass transfer promoter in the hydroformylation reaction [P2].
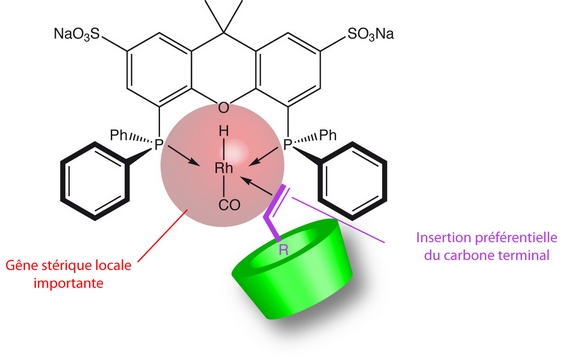
Another possibility consists in using a rigid diphosphine with a strong chelating ability such as sulfoxantphos. We demonstrated that the native β-CD and Rame-β-CD are not capable to decoordinate the diphosphine from the metal.
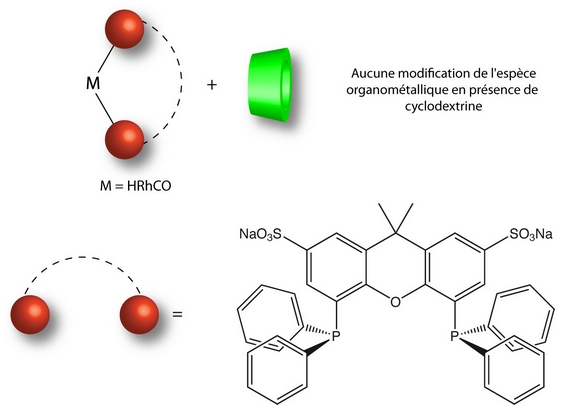 |
The Rame-β-CD / sulfoxantphos association has been studied in the hydroformylation reaction of 1-decene. The presence of Rame-β-CD classically implies an increase in the conversion and chemo-selectivity but also an improvement of the regio-selectivity. This increase in the n/i ratio results both from the inability of Rame-β-CD to decoordinate a phosphorus atom from the rhodium and from the steric hindrance generated by the sulfoxantphos ligand around the rhodium [P1].
 |