



 |
 |
|||||||||||||
 |
||||||||||||||
| Home > Artois Teams > Catalysis and Supramolecular Chemistry > Aqueous organometallic catalysis > Elaboration of catalytic systems without cyclodextrin / phosphine association | ||||||||||||||
CATALYSIS AND SUPRAMOLECULAR CHEMISTRYElaboration of catalytic systems avoiding
|
||||||||||||||
Hydroformylation reaction |
Tsuji-Trost reaction |
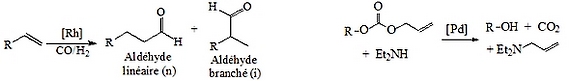 |
|
Figure 1 : Model reactions. |
|
The use of a series of α-CDs has been envisaged. Actually, thanks to their smaller cavity compared to those of β-CDs, α-CDs do not interact with TPPTS (TriPhenylPhosphine TriSulfonate – P(m-C6H4SO3Na)3) the hydrosoluble phosphine used to solubilize the metal. We showed that, during the course of the hydroformylation reaction, high activities and selectivities were obtained in the presence of modified α-CD (methylated or hydroxypropylated) since the intrinsic properties of the catalyst were preserved [P1].
With the aim of generating hydrosoluble organometallic catalysts with phase-transfer properties, we synthesized new α-CDs methylated or not) bearing one or several ammonium groups. The originality lies in the reversible ionic associations (between the TPPTS sulfonates and α-CDs ammonium groups) inducing the formation of catalytic supramolecular entities with CDs acting as second-sphere ligands (Figure 2). The efficacy of these supramolecular systems has been studied in the hydroformylation reaction of decene.
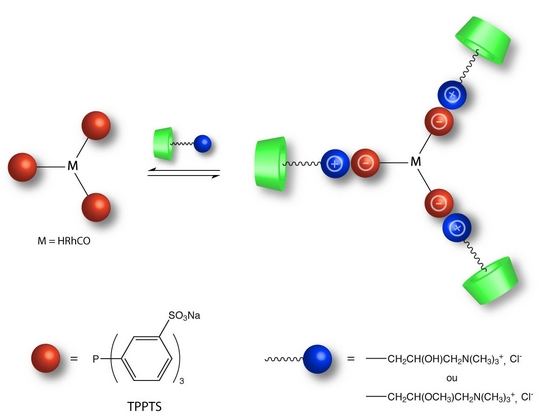 |
Figure 2 : CD-based catalytic supramolecular species |
The conversion and chemoselectivity were improved, as well as interestingly the regioselectivity. This increase has been attributed to the formation of catalytic supramolecular species. As the CD is located close to the coordination sphere of the rhodium, the cavity-included olefin adopts a preferential orientation in favour of the linear aldehyde formation [P2].
Unfortunately, this α-CDs-based strategy is not fully satisfying since a poisoning of the catalytic system is observed during the course of the reaction.