Unité de Catalyse et de Chimie du Solide site Artois
Faculté des Sciences de Lens
PUBLICATIONS MARQUANTES 2009 :
- 3 publications [à Facteur d'impact > 5] :
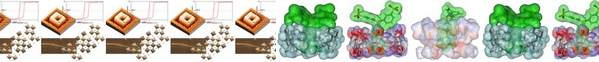
- «Catalytically active nanoparticles stabilized by host–guest inclusion complexes in water»
C. Hubert, A. Denicourt-Nowicki, A. Roucoux, D. Landy, B. Léger, G. Crowyn, E. Monflier
Chem. Commun., 2009, 1228-1230 - doi : 10.1039/b818786j - - IF = 5,5
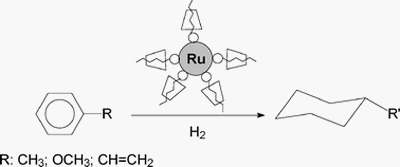 | | Hydrogenation of arene derivatives can be successfully performed in water by using ruthenium(0) nanoparticles stabilized by 1 : 1 inclusion complexes formed between methylated cyclodextrins and an ammonium salt bearing a long alkyl chain. |

- «Ordered arrays of ferroelectric nanoparticles by pulsed laser deposition on PS-b-P4VP(PDP) supramolecule-based templates»
W. van Zoelen, A. H. G. Vlooswijk, A. Ferri, A.-M. Andringa, B. Noheda, G. ten Brinke
Chemistry of Materials 21 (2009) 4719-4723, doi : 10.1021/cm901812j - IF = 5,4
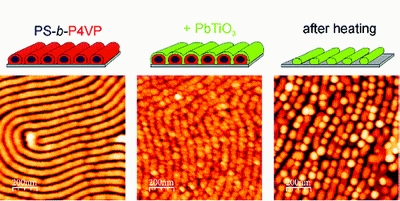 |
| Thin films of comb-shaped supramolecules have been used to create arrays of spatially separated ordered nanorods with a polystyrene core and a poly(4-vinyl pyridine) corona. Room temperature pulsed laser deposition of a uniform layer of lead titanate on top of these nanorod arrays and subsequent heating to 565 °C, far above the degradation temperature of the block copolymer nanorods, resulted in ordered arrays of ferroelectric lead titanate nanoparticles. |

- «Selective discrimination of cyclodextrin diols using cyclic sulfates»
M. Petrillo, L. Marinescu, C. Rousseau, and M. Bols
Org. Lett., 2009, 11 (9), 1983-1985 - IF = 5,4
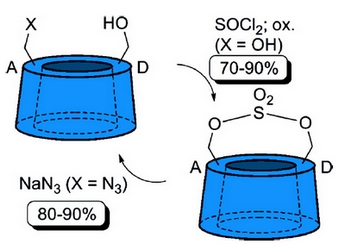 |
A method for selective monofunctionalition of readily available cyclodextrin diols
(2A−F,3A−F,6B,C,E,F-hexadeca-O-benzyl-α-cyclodextrin and 2A−G,3A−G,6B,C,E−G-nonadeca-O-benzyl-β-cyclodextrin) by regioselective nucleophilic opening of their cyclic sulfates is presented. Although the A and D products are nonequivalent in β-cyclodextrin, only the A product is formed. |

Faculté Jean Perrin - rue Jean Souvraz - SP 18 - 62307 Lens Cedex
tel : 03 21 79 17 05
fax : 03 21 79 17 55 |