Unité de Catalyse et de Chimie du Solide site Artois
Faculté des Sciences de Lens
PUBLICATIONS MARQUANTES 2019 :
- 4 publications [à Facteur d'impact > 5]
- «Polymer “ruthenium-cyclopentadienyl” conjugates - New emerging anti-cancer drugs»
T. Moreira, R. Francisco, E. Comsa, S. Duban-Deweer, V. Labas, A-P. Teixeira-Gomes, L. Combes-Soia, F. Marques, A. Matos, A. Favrelle, C. Rousseau, P. Zinck, P. Falson, M-H. Garcia, A. Preto, A. Valente
Eur J Med Chem, 2019, 168, 373-384 - doi: 10.1016/j.ejmech.2019.02.061
IF = 5,573
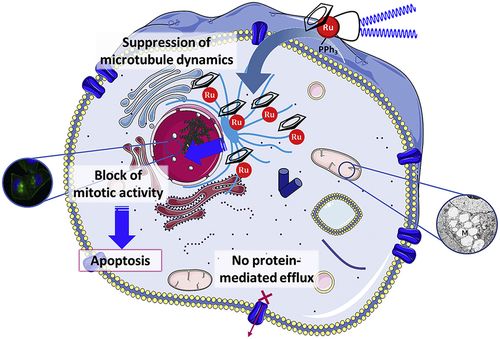 |
| In this work, we aimed to understand the biological activity and the mechanism of action of three polymer-‘ruthenium-cyclopentadienyl’ conjugates (RuPMC) and a low molecular weight parental compound (Ru1) in cancer cells. Several biological assays were performed in ovarian (A2780) and breast (MCF7, MDA-MB-231) human cancer derived cell lines as well as in A2780cis, a cisplatin resistant cancer cell line. Our results show that all compounds have high activity towards cancer cells with low IC50 values in the micromolar range. We observed that all Ru-PMC compounds are mainly found inside the cells, in contrast with the parental low molecular weight compound Ru1 that was mainly found at the membrane. All compounds induced mitochondrial alterations. PMC3 and Ru1 caused F-actin cytoskeleton morphology changes and reduced the clonogenic ability of the cells. The conjugate PMC3 induced apoptosis at low concentrations comparing to cisplatin and could overcame the platinum resistance of A2780cis cancer cells. A proteomic analysis showed that these compounds induce alterations in several cellular proteins which are related to the phenotypic disorders induced by them. Our results suggest that PMC3 is foreseen as a lead candidate to future studies and acting through a different mechanism of action than cisplatin. Here we established the potential of these Ru compounds as new metallodrugs for cancer chemotherapy. |

- «Eggplant-derived biochar- halloysite nanocomposite as supports of Pd nanoparticles for the catalytic hydrogenation of nitroarenes in presence of cyclodextrin»
S. Sadjadi, M. Akbari, B. Léger, E. Monflier, M. Heravi
ACS SUSTAIN CHEM ENG, 2019, 7, 7, 6720-6731 - doi: 10.1021/acssuschemeng.8b05992
IF = 7,632
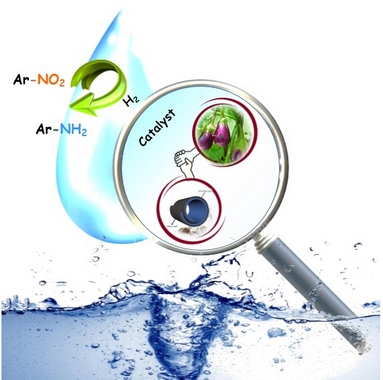 |
| A novel halloysite-hydrochar nanocomposite has been prepared and applied for the immobilization of Pd NPs to furnish an efficient catalyst for the hydrogenation of nitroarenes. It was confirmed that the use of the catalytic amount of β-cyclodextrin (β-CD) could improve the yield of the reaction significantly. With the aim of investigation of the effect of combination of Hal and Char, Char surface modification and the way of use of β-CD on the catalytic activity, several control catalysts were prepared and their catalytic activities were compared with that of the catalyst. It was confirmed that the use of Hal-Char as a support was more effective than the use of each component individually. Moreover, the use of β-CD in its free form was more efficient than incorporating it to the framework of the catalyst or as a capping agent. It was also found that Char in its unmodified form was more efficient than modified ones. To justify the results, a precise study was carried out by comparing the average Pd particle size and loading of each samples. It was confirmed that the Pd particle size and dispersion effectively affected the catalytic activity. Additionally, β-CD amount was a key factor for achieving high catalytic activity. |

- «First evidence of cyclodextrin inclusion complexes in a deep eutectic solvent»
T. Moufawad, L. Moura, M. Ferreira, H. Bricout, S. Tilloy, E. Monflier, M. Costa Gomes, D. Landy, S. Fourmentin
ACS SUSTAIN CHEM ENG, 2019, 7, 6, 6345–6351 - doi: 10.1021/acssuschemeng.9b00044
IF = 7,632
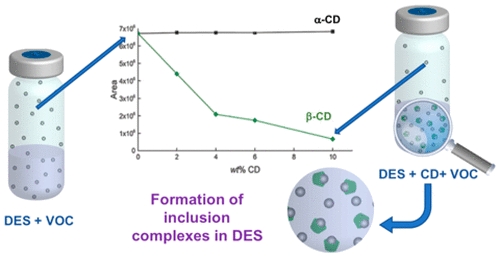 |
| Supramolecular host-guest inclusion complexes based on cyclodextrins (CDs) are generally studied in aqueous solutions or more rarely in the presence of a co-solvent. In this study, we investigate for the first time the ability of CDs to retain their host-guest properties in deep eutectic solvents (DESs). Five cyclodextrins were solubilized in mixtures of choline chloride:urea (ChCl:U) and these new solutions were characterized by measuring their viscosity and density as a function of temperature. The heat of dissolution of β-CD in ChCl:U was measured by calorimetry. The dissolution process is exothermic meaning that the interaction between ChCl:U and CD is favorable (enthalpy of dissolution of – 23.3 J/g). The ability of CDs to form inclusion complexes with various guests in ChCl:U was demonstrated using two different methods: UV-Visible spectroscopy and static headspace gas chromatography. On one hand, the experimental data obtained from the complexation between methyl orange and CDs follows the theoretical fitted curved for 1:1 inclusion complex. On the other hand, the observed volatility reductions of four volatile organic compounds in different mixture of ChCl:U:CD were correlated to the CD/guest formation constant values determined in water. These results will certainly contribute to enlarge the applications of CDs in the DESs field. |

- «Orientation of 4-n-octyl-4’-cyanobiphenyl molecules on graphene oxide surface via electron-phonon interaction and its application in nonlinear electronics»
D. Pratap Singh, B. Duponchel, Y. Lin, J-F. Blach, H. khemakhem, C. Legrand,R. Douali
J MATER CHEM-C, 2019, 7, 2734, - doi: 10.1039/C8TC05696J
IF = 7,059
 |
| We investigate the graphene oxide (GO) dispersed 4-octyl-4'-cyanobiphenyl (8CB) hybrid system in order to reveal the long range molecular interactions. Polarized optical microscopy envisages the vertical orientation of 8CB molecules on the GO surface. The bulk orientation of the 8CB molecules significantly depends upon the concentration of GO. The mechanism of so obtained vertical orientation was further investigated by using Raman and Fourier transformed infrared spectroscopy. We have found that the electron-phonon interaction, between the cyano (CN) functional group of 4-octyl-4'-cyanobiphenyl and phonons of GO, is responsible for the vertical orientation of the 8CB molecules over the GO surface. The role of CN functional group, orientational order parameter and defect intensities have been quantitatively analyzed to support the observations. We have also explored the possibility of integrating the nonlinear electronic applications of the hybrid system by studying the current-voltage (I-V) characteristics. The I-V curves of hybrid system have shown the 103 order higher current in comparison to pristine 8CB material which is due to the combined effect of enhanced charge carrier mobility and the formation of GO networking. |

Faculté Jean Perrin - rue Jean Souvraz - SP 18 - 62307 Lens Cedex
tel : 03 21 79 17 05
fax : 03 21 79 17 55 |