Unité de Catalyse et de Chimie du Solide site Artois
Faculté des Sciences de Lens
PUBLICATIONS MARQUANTES 2010
:
- 7 publications [à Facteur d'impact > 5] dont une distinguée :
- et, 1 publication [à Facteur d'impact < 5] distinguée
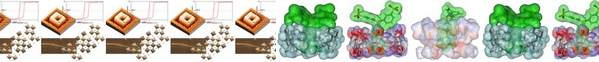
- «Amphiphilic photo-isomerizable phosphanes for aqueous organometallic catalysis»
H. Bricout, E. Banaszak, C. Len, F. Hapiot, E. Monflier
Chem. Commun. 2010, 46, 7813-7815 - doi : 10.1039/C0CC02458A - IF = 5,5
 | | Water-soluble phosphanes were tagged with a light-responding diazo group. Upon UV exposure, the diazo-isomerisation led to phosphane morphology change, resulting in an increase in the reaction rate of an aqueous palladium-catalysed cleavage reaction. |

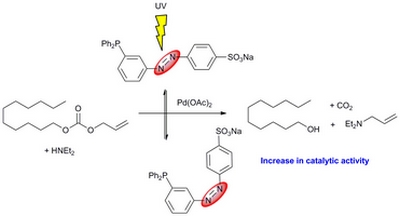
- «Noncovalent functionalization of multiwall carbon nanotubes by methylated-b-cyclodextrins modified by a triazole group»
B. Léger, S. Menuel, D. Landy, J.F. Blach, E. Monflier, A. Ponchel
Chem. Commun., 2010, 46, 7382–7384 - doi : 10.1039/C0CC02761H - IF = 5,5
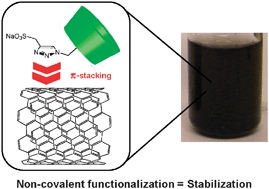 |
| Multiwall carbon nanotubes have been efficiently suspended into water thanks to methylated ß-cyclodextrins (CDs) containing a triazole group, itself substituted in the 4-position by hydrophilic moieties. |

- «New Phosphane based on a β-Cyclodextrin exhibiting a Solvent-Tunable Conformation and its Catalytic Properties»
C. Machut-Binkowski, F.X. Legrand, N. Azaroual, S. Tilloy, E. Monflier
Chem. Eur. J. 2010, 16, 10195 - 10201 - IF = 5,4
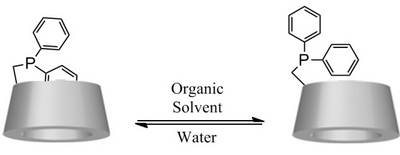 |
| A new diphenylphosphane based on a β-cyclodextrin skeleton that exhibits a dual solubility in water and in organic solvent was synthesised. Interestingly, a solvent-dependent conformation change was evidenced by NMR spectroscopy studies; the self-inclusion of a phenyl group of the phosphane moiety into cyclodextrin cavity observed in water disappeared in organic solvents due to a change in conformation. Hydrogenation or hydroformylation reactions performed in water and in organic solvents showed that this ligand was able to stabilise catalytically active rhodium species in solution. In the case of the hydroformylation reaction, it was demonstrated that regioselectivity was influenced by the solvent-dependent conformation of the ligand. |

- «Ditopic cyclodextrin-based receptors: New perspectives in aqueous organometallic catalysis»
N. Six, S. Menuel, H. Bricout, F. Hapiot, E. Monflier
Adv. Synth. Catal. 2010, 352, 1467-1475 - doi : 10.1002/adsc.201000027 - IF = 5,2
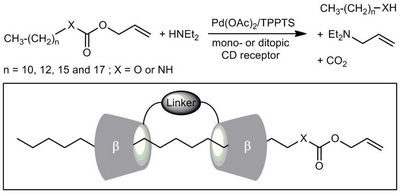 |
| The mass transfer properties of mono- and ditopic β-cyclodextrin-based receptors have been evaluated in a biphasic palladium-catalyzed Tsuji–Trost reaction and compared to one of the best mass-transfer promoters, namely the randomly methylated β-cyclodextrin. While monotopic receptors appeared to be poor mass-transfer promoters of long alkyl chain allyl carbonates or urethanes, cooperative effects have been evidenced with ditopic cyclodextrin-based receptors, opening new perspectives in aqueous organometallic catalysis. |

SELECTED PAPER : «Hottest articles in Green & Sustainable Chemistry»- «Properties and Catalytic Activities of New Easily-Made Amphiphilic Phosphanes for Aqueous Organometallic Catalysis»
M. Ferreira, H. Bricout, N. Azaroual, C. Gaillard, D. Landy, S. Tilloy, E. Monflier
Adv. Synth. Catal. 2010, 352, 1193-1203 - IF = 5,2
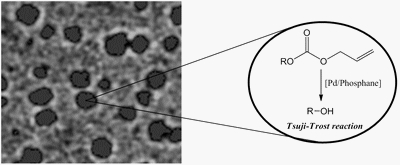 | | Mono - and disulfonated amphiphilic versions of triphenylphosphane (PPh3) and cyclohexyl(phenyl)phosphane were easily synthesized from commercial reagents and sulfuric acid. The behaviour of these phosphanes in solution was investigated by surface tension, isothermal titration calorimetry, nuclear magnetic resonance and cryo-transmission electron microscopy. Two different supramolecular assemblies were evidenced according to the degree of sulfonation. The monosulfonated phosphanes formed well organized micelle-like aggregates while the disulfonated phosphanes formed heterogeneous and disorganized vesicle-like assemblies. The efficiency of these amphiphilic phosphanes was evaluated in the aqueous biphasic, palladium-catalyzed cleavage of allyl alkyl carbonates. |

- «New supramolecular amphiphiles based on renewable resources»
C. Machut, F. Mouri-Belabdellid , J.P. Cavrot, A. Sayede, and E.Monflier
Green Chem., 2010, 12, 772–775 - doi : 10.1039/b924335f - IF = 5,8
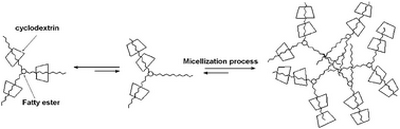 |
| The complexation between esters derived from sorbitol and β-cyclodextrin was studied. Isosorbide dioleate and sorbitan trioleate formed well-defined inclusion complexes with β-cyclodextrin and these inclusion complexes exhibited surfactant behaviour. |

- «Activated Carbon as a Mass-Transfer Additive in Aqueous Organometallic Catalysis Chemistry»
N. Kania, B. Léger, S. Fourmentin, E. Monflier, A. Ponchel
Chem. Eur. J., Vol. 16, Issue 21, June 1, 2010, 6138-6141
doi : 10.1002/chem.201000085 - IF = 5,4
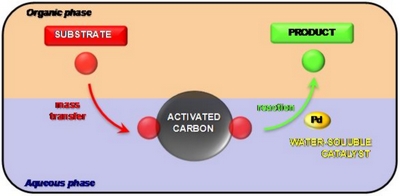 | | Carbon, on active duty : The addition of activated carbon into the reaction medium appears to be a simple and efficient method to solve mass-transfer limitations in aqueous organometallic catalysis (see figure). |

EDITORS CHOICE PAPER
- «β-cyclodextrins modified by alkyl and poly(ethylene oxide) chains: A novel class of mass transfer additives for aqueous organometallic catalysis»
N. Badi, P. Guégan, F.X. Legrand, L. Leclercq, S. Tilloy, E. Monflier
J. Mol. Catal. A. Chem. , 2010, 318, 8-14 - doi : 10.1016/j.molcata.2009.11.015 - IF = 2,9
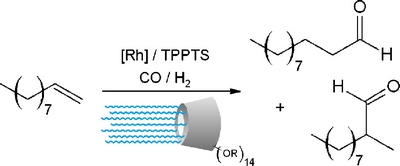 |
| A novel class of β-CDs bearing alkyl chains on the secondary face and poly(ethylene oxide) chains on the primary face was synthesized and their behaviour in rhodium-catalysed biphasic hydroformylation of 1-decene was evaluated. High conversion and selectivities were obtained with a β-CD bearing methyl group on the secondary face and PEO chains on the primary face. |

New Virtual Special Issue - With compliments from the Catalysis Journals |
Dear Prof. Monflier,
I was delighted to see that your very nice paper was chosen as a highlight for the Catalysis Special Issue in which J Mol Cat A featured :
http://mail.elsevier-alerts.com/go.asp?/bESJ001/mAHLAJ3F/u234B7/xLDP1J3F
This is just a quick e-mail to thank for all of your contributions to J Mol Cat A; as both an author and a reviewer,
with kind regards
Professor G. C. Lloyd-Jones FRSC
Editor in Chief
J Mol Cat A. |

Faculté Jean Perrin - rue Jean Souvraz - SP 18 - 62307 Lens Cedex
tel : 03 21 79 17 05
fax : 03 21 79 17 55 |