Unité de Catalyse et de Chimie du Solide site Artois
Faculté des Sciences de Lens
PUBLICATIONS MARQUANTES 2014 :
- 6 publications [à Facteur d'impact > 5]
- «A direct novel synthesis of highly uniform dispersed ruthenium nanoparticles over P6mm ordered mesoporous carbon by host/guest complexes»
N. Gokulakrishnan, G. Peru, S. Rio, J.F. Blach, B. Leger, D. Grosso, E. Monflier, A. Ponchel
J. Mater. Chem. A, 2014, 2, 6641–6648 - doi: 10.1039/C4TA00038B
IF = 7,443
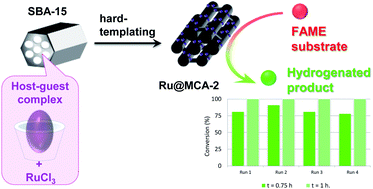 |
| We report a novel concept to prepare a highly ordered mesoporous carbon with a uniform dispersion of ruthenium nanoparticles of 1–2 nm size range using a nano-templating method, based on the combined utilization of a β-cyclodextrin host–guest complex and ruthenium trichloride as respective sources of carbon and metal. The composite material synthesized (Ru@MCA-2) through the polymerization and carbonization of these metallo-supramolecular assemblies possesses high surface area and high pore volume after the removal of the silica template and exhibits high catalytic activity in the hydrogenation of unsaturated fatty acid methyl esters. The reusability of this nanoreplicated catalyst is also demonstrated. |

- «Self-Assembled Metastable γ-Ga2O3 Nanoflowers with Hexagonal Nanopetals for Solar-Blind Photodetection»
Y. Teng, L.X. Song, A. Ponchel, Z.K. Yang, J. Xia
Adv. Mater. 2014, 26, 6238–6243 - doi: 10.1002/adma.201402047
IF = 17,493
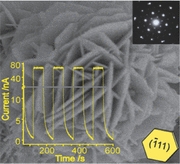 |
| Metastable γ-Ga2O3 nanoflowers assembled from hexagonal nanopetals are successfully constructed by the oxidation of metallic Ga in acetone solution. The nanoflowers with a hollow interior structure exhibit a short response time and a large light-current–dark-current ratio under a relatively low bias voltage, suggesting an especially important potential application in solar-blind photodetection. |

- «Synthesis of 1,4:3,6-dianhydrohexitols diesters from the palladium catalyzed hydroesterification reaction»
R. Pruvost, J. Boulanger, B. Léger, A. Ponchel, E. Monflier, M. Ibert, A. Mortreux, T. Chenal, M. Sauthier
ChemSusChem., 2014, 7, 3157-3163 - doi: 10.1002/cssc.201402584
IF = 7,657
| |
| The hydroesterification of alpha olefins has been used to synthesize diesters from bio-based secondary diols: isosorbide, isomannide, and isoidide. The reaction was promoted by 0.2 % palladium catalyst generated in situ from palladium acetate/triphenylphosphine/para-toluene sulfonic acid. Optimized reaction conditions allowed the selective synthesis of the diesters with high yields and the reaction conditions could be scaled up to the synthesis of hundred grams of diesters from isosorbide and 1-octene with solvent-free conditions |

- «Hydrogen Production by Selective Dehydrogenation of HCOOH Catalyzed by Ru-biaryl Sulfonated Phosphines in Aqueous Solution»
A. Guerriero, H. Bricout, K. Sordakis, M. Peruzzini, E. Monflier, F. Hapiot, G. Laurenczy, L. Gonsalvi
ACS Catalysis 2014, 4, 3002−3012 - doi: 10.1021/cs500655x
IF = 9,312
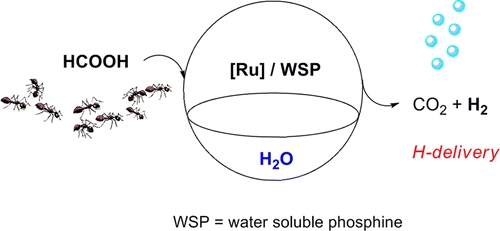 |
| The selective dehydrogenation of aqueous solutions of HCOOH/HCOONa to H2 and CO2 gas mixtures has been investigated using RuCl3·3H2O as a homogeneous catalyst precursor in the presence of different monoaryl-biaryl or alkyl-biaryl phosphines and aryl diphosphines bearing sulfonated groups. All catalytic systems were used in water without any additives and proved to be active at 90 °C, giving high conversions and good TOF values. As an alternative Ru(II) metal precursor, the known dimer [Ru(η6-C6H6)Cl2]2 was also tested as in situ catalyst with selected phosphines as well as an isolated Ru(II)-catalyst with one of them. By using high-pressure NMR (HPNMR) techniques, indications on the nature of the active species involved in the catalytic cycles were obtained. |

- «Low melting mixtures based on β-cyclodextrin derivatives and N,N’-dimethylurea as solvents for sustainable catalytic processes»
F. Jerome, M. Ferreira, H. Bricout, S. Menuel, E. Monflier, S. Tilloy
Green Chem., 2014, 16 (8), 3876 - 3880 - doi: 10.1039/C4GC00591K
IF = 8,020
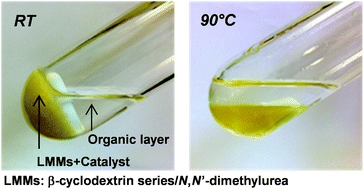 |
| β-Cyclodextrin series and N,N′-dimethylurea formed low melting mixtures able to immobilize organometallic species based on sulfonated phosphanes. Hydroformylation and Tsuji–Trost reactions were efficiently performed in these new solvents which led to new recyclable catalytic systems. |

- «Synergetic Effect of Randomly Methylated β-Cyclodextrin and a Supramolecular Hydrogel in Rh-Catalyzed Hydroformylation of Higher Olefins»
J. Potier, S. Menuel, E. Monflier, F. Hapiot
ACS Catalysis 2014, 4, 2342-2346 - doi: 10.1021/cs5004883
IF = 9,312
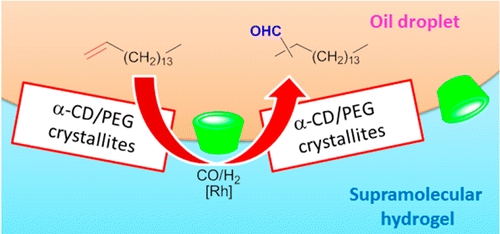 |
| A significant improvement in Rh-catalyzed hydroformylation of very hydrophobic alkenes was achieved using a biphasic catalytic system consisting of a substrate-containing organic phase and a catalyst-containing hydrogel phase [consisting of poly(ethylene glycol) 20000 (PEG20000) and α-cyclodextrin (α-CD)]. The catalytic performance of the Pickering emulsion that resulted from the formation of α-CD/PEG20000 crystallites at the oil droplet surface proved to be greatly dependent upon the presence of additives. We showed that controlled uploads of randomly methylated β-cyclodextrin (RAME-β-CD) within the supramolecular hydrogel could positively affect both the catalytic activity and chemoselectivity of the hydroformylation reaction. Conversely, no Pickering emulsion could be observed using excess RAME-β-CD, resulting in the subsequent degradation of the catalytic performance. Optical microscopy and optical fluorescence microscopy supported the catalytic results and allowed us to explain the role of RAME-β-CD. Indeed, controlled uploads of RAME-β-CD prevented the saturation of the oil droplet surface. RAME-β-CD acted as a fluidifier of the Pickering emulsion and accelerated the dynamics of exchange between the substrate-containing organic phase and the catalyst-containing hydrogel phase. Morever, RAME-β-CD acted as a receptor that participated in the conversion of the alkene by supramolecular means. |

Faculté Jean Perrin - rue Jean Souvraz - SP 18 - 62307 Lens Cedex
tel : 03 21 79 17 05
fax : 03 21 79 17 55 |